Simplified Course of action: BFS removes the need for stoppering and capping stations to the outlet aspect of the filler, simplifying the manufacturing approach and decreasing fees.
(They only prolonged the next amount wherever the BFS was.) The location of your BFS equipment within the POD needed to be strategically chosen since it had to be moved into location.
Some great benefits of Blow/Fill/Seal (BFS) filling technology are recognized. The BFS Superior aseptic approach reduces the need for human intervention in the course of the filling course of action in contrast to standard aseptic filling. Using folks out removes the main contamination resources from the filling surroundings. BFS is largely utilized to manufacture one device dose goods.
A POD is a prefabricated cleanse home which can be transported to the facility, employing a truck, airplane or ship. The space is completely built in a manufacturing facility, with wall panels, doorways, and also some machines and home furnishings, then loaded and delivered. A person vital component not to be missed may be the sheer weight of the BFS program. The bottom or Basis should be robust to guidance it.
We offer the best quality and consistently optimize bottelpack styles for much less Vitality and substance intake as well as warmth recovery.
It is usually Utilized in the foods and pharmaceutical industries for packaging different merchandise. This technology will help automate the packaging approach, rising efficiency, and minimizing the need for manual labor.
In the end the concentrate on is to deliver cost efficient vaccines with the proper immune responses. By minimizing losses in the producing, transport and storage, BFS containers can provide excellent Price advantage.
On top of that, our engineers are creating and tests numerous needle hubs and various components that could be attached directly to the BFS container, enabling inline supply on the drug merchandise without having transfer to some syringe or other external delivery process.
Unither’s Blow-Fill-Seal groups deal with the technology transfer of goods designed by our shoppers or by third get-togethers. They may take care of the complete development of customised medications or health-related units.
Plumbing kits avoid stress loss and the endeavor of procuring all the appropriate fittings to set up an air knife appropriately
One of the aims of the doc will be to determine the bare minimum criteria predicted and to create consistency throughout all buyers of BFS technology for your manufacture of drug goods.
Pharmaceutical and biologics organizations are knowing the many benefits of this technology. With BFS, drug dosing is more precise, and risk of contamination by human intervention is mitigated. This technology is now ever more extra prevalent in the last twenty years since it is more recognized by regulatory companies and the technology has enhanced.
This adaptability makes sure the pharmaceutical industry can keep substantial-high quality benchmarks though Conference the calls for for its products and solutions, presenting affirmation to the liberty and flexibility inherent in BFS technology.
Catalent has get more info carried out in depth exploration into The soundness of biologics going through BFS, to study any probable compatibility problems that the method can have within the molecule or dissimilarities inside the BFS container compared to glass. A comprehensive examine was done using a design monoclonal antibody formulation in its ADVASEPT system, with glass vials with uncoated stoppers utilized as controls.Many parameters of amonoclonal antibody’s-Actual physical Attributes, in addition to balance, potency, and observable click here leachables have been testedand measured in excess of a nine month time period.
 Tatyana Ali Then & Now!
Tatyana Ali Then & Now!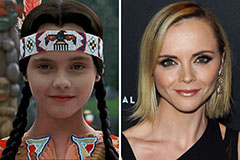 Christina Ricci Then & Now!
Christina Ricci Then & Now! Michael Jordan Then & Now!
Michael Jordan Then & Now! Daryl Hannah Then & Now!
Daryl Hannah Then & Now! Robin McGraw Then & Now!
Robin McGraw Then & Now!